

 |
 |
| Fact Sheet | Radiation at FUSRAP Sites | August 1998 |
| Return to list of Fact Sheets | home |
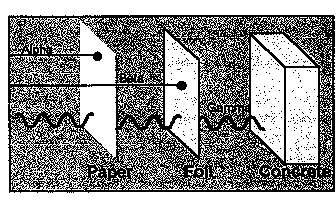
Radiation cannot be seen, heard, smelled, or tasted. However, it can be detected and measured by instruments such as Geiger counters, dosimeters, and similar devices. Levels of radiation are expressed in several different units. One of the most useful is the rem, which measures radiation dose in terms of its potential health effects on persons who might be exposed to it.
Small amounts of radiation dose are expressed in millirems (thousandths of a rem), abbreviated as mrem. For example, a chest X-ray produces a dose of about 40 mrem, a back X-ray about 3,000 mrem, and a dental X-ray about 150 mrem.
The amount of radiation that can leave the boundaries of FUSRAP sites is kept to levels as low as reasonably achievable. The exposure a member of the general public can receive as a result of radiation from FUSRAP sites is very low. The maximum allowable exposure is 100 mrem per year above background levels. By comparison, the average American receives about 360 mrem per year from background radiation and medical exposure.
Natural and synthetic substances that emit radiation are called radioactive materials. Many buildings contain naturally occurring radioactive materials. For example, radioactive elements in the granite in the U.S. Capitol Building emit radiation producing an exposure of about 85 mrem per year. The human body itself contains substances that contribute about 11 percent of the average annual radiation exposure.
Some consumer products are also sources of radiation. A person who smokes two packs of cigarettes per day receives 8,000 mrem per year. Smoke detectors produce about l/l 00 mrem per year. Certain household appliances such as color television sets and microwave ovens also produce very small amounts of radiation. On the average, consumer products account for about 3 percent of our annual exposure.
Several sites with industrial contamination similar to that produced by MED or AEC activities have also been added to FUSRAP by Congress. The radioactive residues at FUSRAP sites consist mostly of forms of the elements uranium, thorium, and radium that emit low levels of radiation. FUSRAP was established to ensure that the public and the environment are not exposed to potentially harmful levels of radiation from these sites. The goal of FUSRAP is to clean up or contain the radioactive material so that the sites may be released for appropriate future use.
Return to the top of the page
Return to list of Fact Sheets